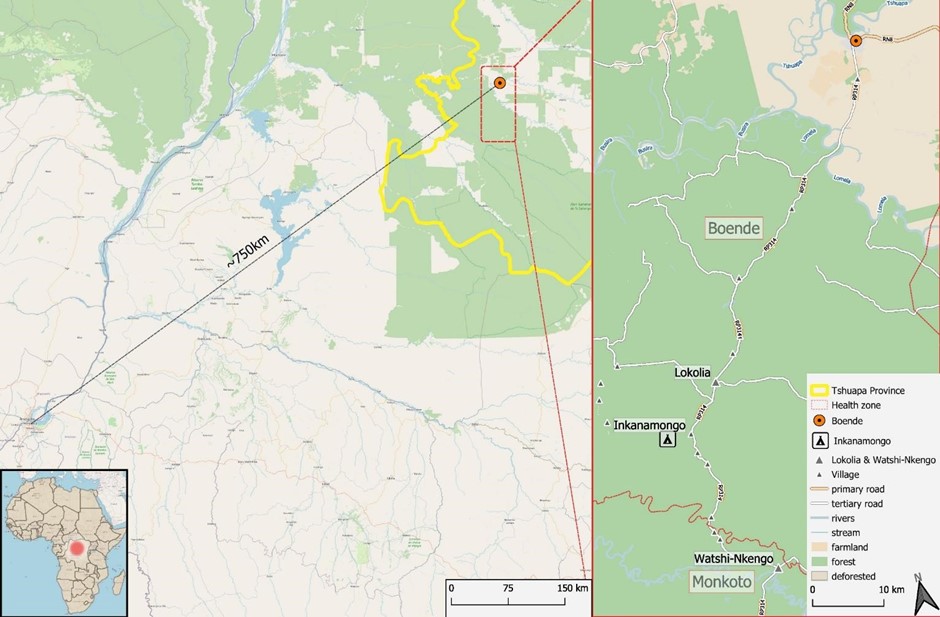
Public health, policy and practice – impact of the Boende EBOVAC clinical trial in the Democratic Republic of Congo (DRC)
This study is taking place alongside the Phase 2 clinical trial (EBL2007), set up in an Ebola-endemic region to evaluate the immunogenicity and safety of the two-dose Ebola vaccine regimen in health care providers (HCPs). It aims to explore the impact of the trials on the staff, community, and participants, looking at the roles they have played, challenges faced and how these were overcome, internal and external collaboration and communication, hopes for the future of the site, experiences of the trial procedures, and views about healthcare services and routine vaccinations.
The study aims to describe how the trial has transformed participants’ perceptions of medical research, state healthcare services, and biomedical citizenship. At a policy level, the development and use of an algorithm for the management and support of Non-Related (Serious) Adverse Events is also being investigated while being implemented.
Comparative Case Studies: Boende (EBL2007) and Goma (EBL3008) trials
Linked to the study on the material and social legacies of the Phase 2 clinical trial (EBL2007), this comparative case study between the Boende and Goma trials, which are administrating the same two-dose Ebola vaccine regimen at the population level, is exploring why and how the different decisions made in these two trials are being perceived, debated and contested.
The topics covered include:
- Inclusion/exclusion of pregnant-lactating women for Ebola vaccination;
- People’s motivations to participate to these trials;
- Concerns and rumours about the vaccine or the trials;
- Dynamics/relationships between the clinical trial staff and different trial participants (such as Health Care Workers, children and pregnant women).
Community Preparedness and Acceptance of Vaccination Strategies
This study has two inter-related components. The first focuses on the community experiences and perceptions of the 7th Ebola outbreak in the DRC (2014) and the international response at that time, as well as ongoing actions by local and traditional authorities to improve preparedness for future outbreaks. The aim of the study is to gather different views from the community, which will contribute to a growing body of literature exploring how social processes during the implementation of medical emergency interventions shape, and are shaped by, local socio-economic and political contexts.
The second study component is exploring perceptions and acceptance of various vaccination strategies among different populations in the DRC.
Local Ebola Virus – Ecosystem – Livelihood dynamics
Most human Ebola outbreaks start from a spill-over event from wildlife. Ebola emergence in forest regions such as Boende and the surrounding area, is shaped by the interplay of climatic, social-economic and ecological dynamics. This study aims to understand how people’s livelihoods and interactions with ecosystems and animals affect their exposure to diseases. It also looks at how to co-construct an integrative model of contact patterns that are more representative of the study setting by gathering qualitative and quantitative data on Ebola–ecosystem–livelihood dynamics.
Regulatory experience relating to vaccine trials during an epidemic
In the DRC, new standards have been put in place by national and provincial institutions following the experience of the four most recent Ebola epidemics in the country (2017-2020), during which the administration for compassionate use of unlicensed vaccines was actually carried out or initiated.
This study aims to:
- describe the experience of the various directives and regulations taken by the Congolese authorities regarding vaccine trials and deployments since 2014 and during the last EVD outbreaks in the Province of Equateur;
- identify and describe whether and how the experiences of ethical and regulatory authorities during the West African epidemic influenced current guidelines and practices in the DRC;
- review laws, regulations and standards relating to the importation, evaluation and deployment of vaccines at the national and provincial levels of the country, including mapping the flow of decision-making and planning within the health system in relation to clinical trials; and
- identify what was easy to implement, as well as challenges faced, and analyse the reasons for resistance to changes in standards and regulations, in order to propose solutions to prevent and respond to future outbreaks.
Social science study on acceptability and feasibility of the Iris scan
EBOVAC3 aims to use biometric identification (iris scanning) to uniquely identify healthcare provider (HCP) vaccine recipients. The iris scanning tool utilises Connect for LifeTM technology, which links HCP identification with the clinical trial visit schedule and sends out mobile messages as visit reminders to decrease drop-out rate for the two-dose EBOVAC3 vaccination schedule.
This qualitative study assesses how HCPs respond to iris scanning as a means of identification and investigates the feasibility and acceptability of mobile phone messages as visit reminders in the rural context of Tshuapa.
Mapping of Preparedness Activities and Gap Identification
This study aims to build on an existing outbreak preparedness map of the Tshuapa Province in 2018-2019, produced by other projects (Ecole de santé publique de Kinshasa and CDC), through exploring community governance experiences and responses to the Ebola epidemic, as well as preparedness for future outbreaks. The research adopts a One Health approach, including surveillance structures of animal, human and environmental health and the communication between them.
This will also compliment the first study in this section by building a picture of the institutional legacy of the Ebola outbreak and by mapping current mechanisms via which communities might respond to an outbreak, as well as the partnership frameworks through which a vaccine could be rapidly deployed to reach affected populations.
Publications
Zola Matuvanga T, Johnson G, Larivière Y, Esanga Longomo E, Matangila J, Maketa V, Lapika B, Mitashi P, Mc Kenna P, De Bie J, Van Geertruyden JP, Van Damme P, Muhindo Mavoko H. (August 2021). Use of Iris Scanning for Biometric Recognition of Healthy Adults Participating in an Ebola Vaccine Trial in the Democratic Republic of the Congo: Mixed Methods Study. J Med Internet Res. 23(8):e28573. doi: 10.2196/28573.
Lemey G, Larivière Y, Zola TM, et al. (June 2021). Algorithm for the support of non-related (serious) adverse events in an Ebola vaccine trial in the Democratic Republic of Congo. BMJ Global Health. 6(6). doi:10.1136/bmjgh-2021-005726